Salicylic acid's chemical structure enables exfoliation, pore cleansing, and anti-inflammatory action.
Salicylic acid has been known for centuries, but few people understand the power behind its molecular structure. It is a simple beauty ingredient and a powerful tool in treating many complex dermatological diseases.
The unique chemical structure of salicylic acid directly determines the biological effects it brings. This nature's thorough exploration helps enhance medical applications, opening new doors for modern skin care solutions.
Salicylic acid's chemical structure enables exfoliation, pore cleansing, and anti-inflammatory action.
Basic Chemical Profile
Salicylic acid is a naturally occurring organic compound belonging to the phenolic acid group. Its simple structural basis but outstanding biological function allows it to interact with many complex physiological systems and biological mechanisms in human skin.
Correct IUPAC Name
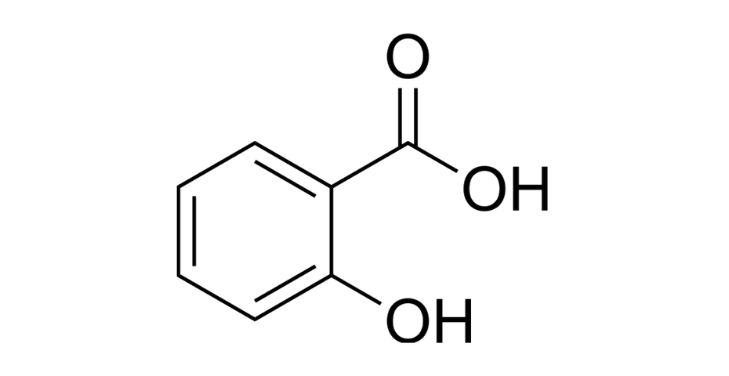
Salicylic acid is named according to the IUPAC nomenclature, 2-hydroxybenzoic acid. This name correctly reflects the position of the hydroxyl group (-OH) attached to carbon number 2 of the benzene ring, while the carboxylic acid group (-COOH) holds a fixed position at carbon number 1. This numbering highlights the spatial orientation of the molecule in biological reactions.
Formula and molecular weight
The molecular formula of salicylic acid is C₇H₆O₃, showing that it consists of seven carbon atoms, six hydrogen atoms, and three oxygen atoms. The molecular weight is approximately 138.12 g/mol, light enough to diffuse easily through the epidermis but still large enough to remain stable in biological environments.
Basis for biological activity
This structure is the basis for salicylic acid to perform various therapeutic functions, from deep cleansing to regulating skin inflammation. It exists not only as a chemical, but also as an essential part of the modern dermatological medical treatment arsenal.
Chemical Structure Breakdown
The molecular structure of salicylic acid is a masterpiece of neat chemistry. Each functional group is arranged with a clear biological purpose in mind. Strong covalent bonds in the aromatic ring provide molecular stability, while the substituents provide the functional flexibility needed in the physiological environment.
The benzene ring as a scaffold
The six-membered benzene ring acts as a rigid scaffold, precisely holding the functional groups in space. This is essential for maintaining stability and the ability to interact at the microscopic level.
Two dominant functional groups
Salicylic acid carries two polar functional groups: a hydroxyl (-OH) attached to carbon two and a carboxylic acid (-COOH) attached to carbon one on the benzene ring. This proximity creates intramolecular hydrogen bonds, increasing chemical stability and the ability to penetrate biological membranes.
BHA Classification – Beta Hydroxy Acids
The structure with a hydroxyl group attached to the beta position (adjacent to the acid group) establishes salicylic acid as a beta-hydroxy acid (BHA). This is the key to its lipid-penetrating action and deep penetration into pores, where other acids cannot effectively intervene.
How Structure Influences Its Function
The chemical structure of salicylic acid not only determines its physical properties but also holds the key to its specific biological mechanisms of action. Combining the aromatic ring, hydroxyl group, and carboxylic acid group creates a versatile functional platform, allowing the compound to interact effectively with skin tissues and cellular structures.
Lipid Solubility
With its semi-polar nature and oil solubility, salicylic acid easily penetrates sebum and reaches deep into the hair follicle. This allows it to clear blockages, reduce pore blockages, and aid in the resolution of comedones.
Hydroxyl Group – Anti-Inflammatory and Exfoliating Agent
The -OH group at the ortho position contributes to its anti-inflammatory properties by inhibiting cyclooxygenase (COX) and loosening the bonds between old keratinocytes, allowing for effective and gentle exfoliation.
Carboxylic acid enhances the keratolytic effect
The COOH group allows salicylic acid to dissociate ions in the skin's low pH environment. This supports the breakdown of keratin bridges and promotes the removal of dead skin cells without causing deep damage. This is the core factor in the ability to regenerate and improve skin structure.
Comparison to Other Acids
Each acid that acts on the skin has a distinct chemical nature, determining its action mechanism and range of applications. Salicylic acid, with its beta hydroxy acid (BHA) structure, exhibits a deep, selective action, and is suitable for oily, folliculitis, or hyperkeratosis skin.
Polarity and Penetration
Salicylic acid is lipid-soluble, easily bypassing the sebum layer and penetrating deep into the pores. This ability creates a cleansing and anti-inflammatory effect from within—a clear difference from water-soluble acids such as alpha hydroxy acids (AHAs).
Specific epidermal interaction
AHAs such as glycolic or lactic acid primarily act on the skin's surface, accelerating the rate of keratinocyte shedding in the outermost layer. Meanwhile, salicylic acid has an affinity for keratin in deeper epidermal cells, supporting sebum removal, keratolytic, and sebaceous gland activity.
Treatment orientation according to skin type
Salicylic acid is advantageous in oily skin, acne, and keratosis pilaris. The molecular structure determines the independent treatment role, guiding the selection of cosmeceuticals according to each skin type.
Derivatives and Related Compounds
Salicylic acid is an important chemical precursor in synthesizing many biologically active compounds. When undergoing esterification or acylation reactions, this molecule produces derivatives with various applications, from pain relief to treating dermatitis and musculoskeletal pain.
Aspirin – acetylation of the hydroxyl group
Aspirin (acetylsalicylic acid) is formed when the hydroxyl group (-OH) of salicylic acid is acetylated. This derivative exhibits anti-inflammatory, analgesic, and antipyretic properties through the irreversible inhibition of the cyclooxygenase enzyme, reducing prostaglandin synthesis. This structural basis establishes the role of aspirin in cardiovascular medicine and the treatment of chronic arthritis.
Methyl salicylate – an ester form that is easily absorbed through the skin
Methyl salicylate is the ester of salicylic acid and methanol. This volatile compound is commonly found in topical preparations due to its local warming properties and ability to relieve muscle pain. Methyl salicylate readily penetrates the epidermis and acts as a vasodilator, increasing local blood flow.
Pharmacologically Directed Derivatives
Each modification of the salicylic acid molecule is a chemical manipulation and a strategic pharmacological transformation, transforming a natural compound into a wide range of applications in internal medicine and dermatology.
Applications Tied to Its Structure
The unique molecular structure of salicylic acid is the basis for a wide range of biologically precise medical and dermatological applications. The spatial arrangement of the functional groups on the benzene ring shapes the specialized treatment mechanism suitable for each specific pathological manifestation on the skin.
Acne treatment
Salicylic acid penetrates the lipid layer of the sebaceous gland, penetrates the pore, and exfoliates the blockage. Thanks to the hydroxyl group, it is anti-inflammatory in the inflamed hair follicle and helps soothe irritated tissue. The moderately polar structure facilitates deep but controlled action.
Wart and callus removal
With its powerful keratolytic activity from the -COOH group, salicylic acid breaks down the protein bonds in the thick stratum corneum. The ion dissociation in the acidic environment promotes the exfoliation of each layer of dead cells, effectively treating warts, calluses, and localized hyperkeratotic lesions.
Scalp pathology support
Salicylic acid acts as a gentle exfoliant and stabilizes the microflora in preparations for treating dandruff, seborrheic dermatitis, and scalp psoriasis. Thanks to its selective permeability and keratin conditioning properties, it helps to reduce flaking and visibly soften the scalp.
Conclusion
Salicylic acid is a chemical compound with a unique molecular structure that is central to many modern medical and skin care applications. The molecular design with a benzene ring and two hydroxyl and carboxylic acid groups functions to create a deep and precise mechanism of action.
This structure not only determines the ability to appraise and interact with cells but also opens up diverse treatment potential, from acne to complex hyperkeratosis. Thanks to its outstanding effectiveness and level, salicylic acid continues to affirm its important position in dermatology.
Related Articles
Frequently Asked Questions (FAQs)
- Can Salicylic Acid Cause Skin Irritation? – Salicylic acid is gentle on most skin types, but people with sensitive skin should first do a patch test to avoid irritation or overdrying.
- Why is salicylic acid effective in treating acne? – Salicylic acid's oil-soluble properties allow it to penetrate deep into pores, clearing sebum, reducing inflammation, and preventing blockages—the main causes of acne.
- Is salicylic acid suitable for dry skin? – Dry skin can use salicylic acid at a low concentration and combine it with moisturizer to avoid unbalancing the skin barrier.
- How does salicylic acid work in treating warts? – The carboxylic acid group breaks down the protein bonds in the thick stratum corneum, helping to exfoliate dead cells and effectively remove warts.
- How is salicylic acid different from alpha-hydroxy acids? – Salicylic acid is an oil-soluble beta-hydroxy acid that penetrates deep into pores, while alpha-hydroxy acids are water-soluble and act primarily on the skin's surface.

